This article is part of A\J’s web series Night School. In celebration of back-to-school time and our Night issue, the A\J web team brought you a series of quick lessons, posted between September 16 to October 11, 2013, covering everything from activism tactics and canning tips to how factory farms breed disease.
This article is part of A\J’s web series Night School. In celebration of back-to-school time and our Night issue, the A\J web team brought you a series of quick lessons, posted between September 16 to October 11, 2013, covering everything from activism tactics and canning tips to how factory farms breed disease.
It’s no secret that the way we do agriculture matters for our health. But there’s more to think about than just getting a balanced diet. We’re inadvertently raising new diseases along with our meat by creating the conditions for viruses and bacteria to evolve.
The Resistance
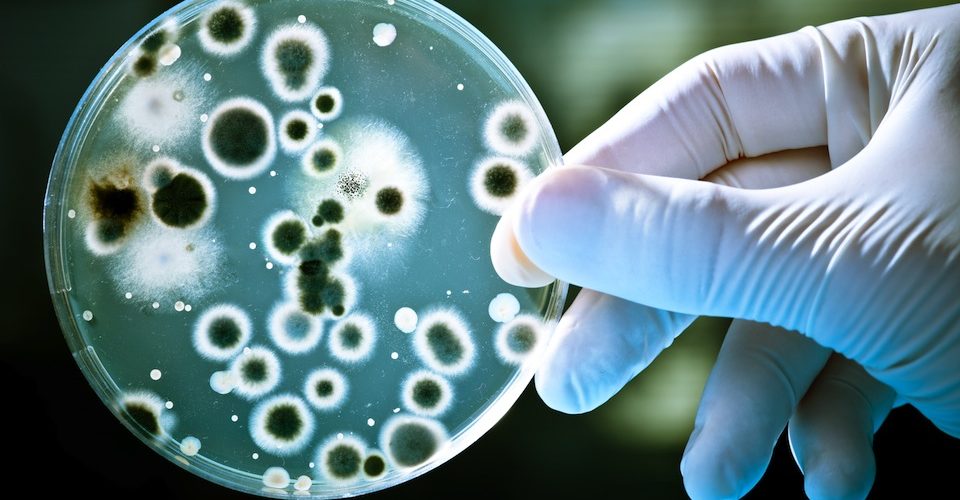
When Alexander Fleming came back from vacation in 1928 and started cleaning up the Petri dishes he’d left out on his workbench, he saw something that revolutionized medicine. Fungus had gotten into one of the plates of Staphylococcus bacteria, and where the mould grew, the bacteria died.
Reasoning that some molecule in the “mould juice” had the power to kill bacteria, he tested it on a number of diseases. The molecule, which he named penicillin, turned out to fight syphilis, scarlet fever, pneumonia, meningitis and diphtheria.
Since then, scientists have made over 100 antibiotics that target important things bacteria have to do to survive. For example, penicillin interferes with the cell wall shared by many bacteria, while tetracycline shuts down the protein synthesis machinery in other species.
But, according to the US Centers for Disease Control, our misuse of these wonder drugs means that we’re on the verge of “a post-antibiotic era.”
We’ve been experimenting on bacteria for over a century, watching them evolve incredible new solutions to problems they face. In fact, Fleming himself warned about resistance in the 1940s, and yet we were and still are careless in our use of them.
The swarms of bacteria that infect people are not all identical cells. As they divide, they pick up mutations, and some mutations make them a little more resistant to antibiotics. If antibiotics are prescribed and taken correctly, they should team up with your immune system to wipe out an infection completely, even the stubborn bacteria with some resistance.
However, if the course of treatment is stopped before the toughest bacteria are killed, then they can make a comeback, and all their offspring will be the resistant kind, too. They can also, in some cases, transfer their resistance genes to other kinds of bacteria, so even if they are eventually hunted down by your immune system, the resistance genes are still out there, ready to strike again. This is why you’re always instructed by your doctor to finish a round of antibiotics, even if you feel better!
In mid-September, the CDC released a report condemning the flippant use of antibiotics, naming farms as one place where this happens. It has to be emphasized that farms are absolutely not the biggest cause of antibiotic resistant bacteria. Hospitals are by far the worst offenders, and the report describes what can be done about that. However, the CDC does speak to our topic directly:
Antibiotics are also commonly used in food animals to prevent, control, and treat disease and to promote the growth of food-producing animals. Drug-resistant bacteria can remain on meat from animals. When not handled or cooked properly, the bacteria can spread to humans. The use of antibiotics for promoting growth is not necessary, and the practice should be phased out. Recent guidance from the US FDA describes a pathway toward this goal.
In Canada, our regulations are even worse. Last year, the Canadian Medical Association Journal put out an article that was even more damning than the CDC report. Here’s a taste: “Meat production is currently regulated under provincial laws that fail to address the growing problem with antibiotic resistance driven by drug misuse.”
For more on antibiotic resistance, check out this excellent coverage of the CDC report from Wired.
The Pandemic Cocktail
The flu evolves so fast that there’s a major new strain every year.
The virus is a tiny spiky capsule with 8 strips of RNA. It uses one type of its spikes (the surface protein Hemagglutinin) to infect animal cells. The virus heads to the nucleus, where its RNA pieces are used as templates for new virus particles. Then it uses its other surface protein (Neuraminidase) to break out and infect new cells. Different versions of these are what get the virus names like H1N1 or H2N2.
If an animal like a pig or human is unlucky enough to catch both of those strains at the same time, then some of its cells will be infected by both H1N1 and H2N2. When it comes to wrapping up the new virus particles, a few of the 8 RNA pieces will get swapped between strains. Now the pig (or human) is contagious for at least 3 kinds of flu: H1N1, H2N2, H1N2 and any other combinations that got produced (there are 64 possible combinations).
This mixing is called re-assortment, and the more animals you pack into one area, human or otherwise, the higher the chance of this happening.
The 2009 H1N1 pandemic evolved in this way. It contained RNA pieces from human influenza, avian influenza and two kinds of swine influenza. The odds of it making the jump to humans increase on farms where huge numbers of the animals come into regular contact with humans.
Of course, all of this can happen without factory farms. The “Spanish Flu” of 1918 that infected one third of the human population is a good reminder. However, our current agricultural system stacks the odds in favour of more re-assortment happening in the presence of humans.
We could also be doing a lot more to track strains of flu as they move and mutate through our agricultural system; sending breeder pigs around the world gives the flu virus even more opportunity.
This is not a disease that needs helping, so we need to keep tabs on the ways that we are making bacterial resistance possible, and ideally stop the problems before they spiral out of control.
Ben is a former A\J editorial intern. He’s currently a freelance writer based in Ottawa. He tells stories about nature, science and policy.
Ben is a former A\J editorial intern. He’s currently a freelance writer based in Ottawa. He tells stories about nature, science and policy.